Skeletal Muscle
Table of Contents
What is Skeletal Muscle?
Commonly referred to as muscles, skeletal muscles are organs of the vertebrate muscular system, usually linked to a skeleton’s bones via tendons. Skeletal muscle cells, sometimes referred to as muscle fibers, are considerably longer than those of other forms of muscular tissue.
Because of how the sarcomeres are arranged, the muscular tissue of skeletal muscles is striated, giving the appearance of stripes.
The somatic nervous system regulates the voluntary muscles found in the skeleton. The remaining muscle tissue is divided into two categories: smooth muscle, which lacks striating, and cardiac muscle, which has to striate; both are categorized as being involuntary, or controlled by the autonomic nervous system.
Several fascicles, or bundles of muscular fibers, make up a skeletal muscle. Every muscle and every fiber is encircled by a layer of fascia, a form of connective tissue.
Myogenesis is the process by which developing myoblasts fuse to generate lengthy, multinucleated cells that eventually become muscle fibers.
The nuclei of these cells are situated inside the cell membrane and are referred to as myonuclei. To satisfy their energy demands, muscle fibers also have many mitochondria.
Myofibrils are the building blocks of muscle fibers. Actin and myosin filaments, or myofilaments, are the fundamental functional, contractile units of the muscle fiber required for muscular contraction. These filaments are repeated in units termed sarcomeres, which make up the myofibrils.
Although the oxidation of lipids and carbohydrates powers muscles primarily, anaerobic chemical processes also power muscles, especially in the case of quick twitch fibers.
Adenosine triphosphate (ATP), which powers the movement of the myosin heads, is produced by these chemical processes.
Human skeletal muscle makes up around 35% of the body weight. Skeletal muscle is responsible for generating movement, preserving posture, regulating body temperature, and stabilizing joints. Additionally, skeletal muscle is an endocrine organ.
Skeletal muscle secretome contains subsets of 654 distinct proteins, lipids, amino acids, metabolites, and short RNAs under various physiological circumstances.
The majority of multinucleated contractile muscle fibers make up skeletal muscles. Skeletal muscles do, however, also include a sizable population of resident and invading mononuclear cells.
Myocytes comprise the vast bulk of skeletal muscle in terms of volume. Skeletal muscle myocytes often measure 2-3 cm in length and 100 µm in diameter, making them incredibly big cells.
Muscle cells have substantially smaller mononuclear cells in contrast. A few of the mononuclear cells found in muscles include neutrophils, macrophages, and endothelial cells.
The muscle fiber cells, or myocytes, are the subject of much skeletal muscle research; these are covered in depth in the initial portions of the sections that follow.
But more recently, attention has also been drawn to the many skeletal muscle mononuclear cell types and the endocrine activities of muscle, which are discussed below.
Structure
Gross anatomy
The human body comprises around 600 skeletal muscles, accounting for approximately 40% of a healthy young adult’s total weight. Men typically have around 61% greater skeletal muscle than women in Western countries.
Most muscles are found in bilateral pairs that service the body’s two sides. Often, muscles are categorized as groups of muscles that cooperate to act. There are numerous primary muscle groups in the torso, such as the pectoral and abdominal muscles; the hand, foot, tongue, and extraocular muscles of the eye are subdivided into intrinsic and extrinsic muscles.
Additionally, muscles are divided into compartments. There are four muscles in the arm and four in the leg.
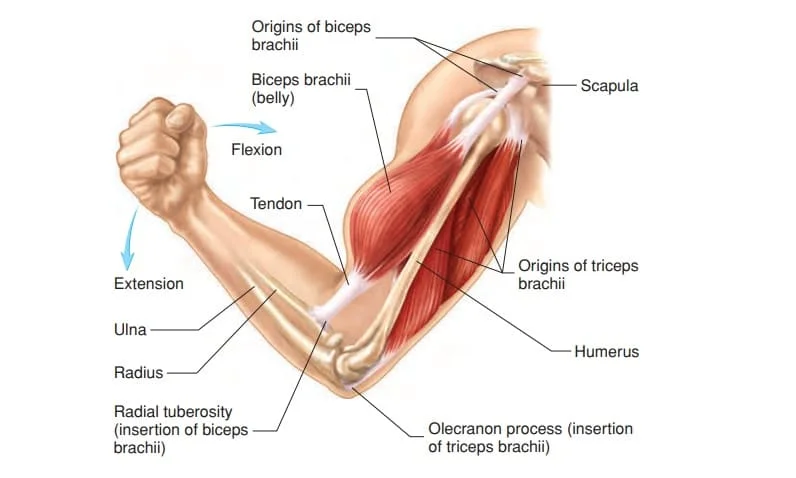
A muscle has a non-contractile portion of thick fibrous connective tissue that makes up the tendon at either end in addition to the contractile portion made up of the muscle’s fibers.
Skeletal movement is provided by the tendons connecting the muscles to the bones. Tendons are included in the length of a muscle.
Deep fascia, or connective tissue, is found in every muscle. Each muscle fiber is called an endomysium, each muscular fascicle is called a perimysium, and each muscle is called an epimysium. Deep fascia is specialized inside muscles.
Mysia is the collective term for these levels. Additionally, muscular groups are divided into muscle compartments by deep fascia.
Muscle spindles and Golgi tendon organs are two different types of sensory receptors that are present in muscles.
Stretch receptors called muscle spindles are found in the muscular belly. Proprioceptors at the myotendinous junction called Golgi tendon organs sense the tension in a muscle.
Skeletal muscle cells
The individual contractile cells that make up a muscle are known as skeletal muscle cells or muscle fibers. About 253,000 muscular fibers may be found in a single muscle, such as the biceps of a young adult male.
The only multinucleated muscle cells having what is known as myonuclei are skeletal muscle fibers. This happens when myoblasts fuse, each of them donating a nucleus, during myogenesis.
Myomaker and Myomerger, two proteins known as fusogenic that are particular to muscles, are essential for fusion.
The skeletal muscle cell requires a lot of nuclei to create the vast quantities of proteins and enzymes required for the cell to function normally. Nuclei can number in the hundreds or thousands inside a single muscle fiber. Up to 3000 nuclei can be found in a muscle fiber, for instance, a 10 cm-long muscle fiber in the human biceps.
In contrast to non-muscle cells, which have their nucleus positioned in the center, the myonucleus is elongated and situated near the sarcolemma. Each nucleus has its myonuclear domain, which is in charge of sustaining the amount of cytoplasm in that specific region of the myofiber. The myonuclei are organized quite equally along the fiber.
Between the muscle fiber’s sarcolemma and basement membrane is a population of muscle stem cells called microsatellite cells, or satellite cells.
Normally dormant, these cells can be stimulated by disease or exercise to produce more myonuclei for muscle development or repair.
Attachment to tendons
The musculotendinous junction, often referred to as the myotendinous junction, is a complicated interface zone where muscles link to tendons.
This location is specifically designed for the major transfer of force. Force is transferred from the sarcomeres in the muscle cells to the tendon at the muscle-tendon junction.
Tendons and muscles grow together closely during development, and once they unite at the myotendinous junction, they function as a dynamic unit to transfer force from a muscular contraction to the skeletal system.
Arrangement of muscle fibers
The arrangement of muscle fibers concerning the axis of force generation—which extends from a muscle’s genesis to its insertion—is referred to as muscular architecture. Types of parallel and pennate muscle are the typical configurations.
The axis of force generation is parallel to the fascicles in parallel muscles, yet there can be variations in the way the fascicles are related to each other and their tendons. The fusiform, strap, and convergent muscles exhibit these differences. The fibers of a convergent muscle converge at the insertion and spread widely at the origin, giving the muscle a triangular or fan-like form.
A circular muscle, like the orbicularis oculi, whose fibers are longitudinally oriented yet form a circle from origin to insertion, is a less common example of a parallel muscle. The stress that a muscle may produce between its tendons can vary depending on these various topologies.
Pennate muscle fibers are oriented perpendicularly to the axis of force production. Given that it is pulling off-axis, this pennation angle lowers the effective force of any one fiber.
However, due to this angle, the physiological cross-sectional area (PCSA) increases since more fibers may be crammed into the same muscle volume.
The fiber packing effect more than compensates for the off-axis orientation’s efficiency loss in terms of force production. The overall pace of muscle shortening and the total excursion are the trade-offs.
Both the total length of shortening and the overall muscle shortening pace are lower than the fiber shortening speed.
Muscle fiber growth
When muscles are used, they grow, and when they are not, they shrink. This is because exercise causes an increase in myofibrils, which in turn causes an increase in the total size of muscle cells.
In addition to growing larger, well-trained muscles can produce more mitochondria, myoglobin, glycogen, and capillary density.
However, muscle cells are not able to proliferate to create new ones, which means that an adult has fewer muscle cells than a baby.
Muscle naming
naming of muscles involves the use of several terminology about their size, form, activity, location, orientation, and number of heads.
By size
Brevis indicates short, longus indicates long, longissimus indicates longest, magnus indicates large, major indicates larger, Maximus indicates largest, minor indicates smaller, minimus indicates smallest, latissimus indicates broadest, and vastus indicates enormous.
These words, like gluteus maximus and gluteus minimus, are frequently used after the specific muscle.
By relative shape
Serratus means saw-toothed; orbicular means circular; pectinate means comblike; piriformis means pear-shaped; platys means flat and gracilis means slender.
Triangular objects are called deltoid, four-sided objects are called quadratus, rhomboideus is a term for a rhomboid form, round or cylindrical objects are called teres, and trapezoids are called trapezoids. Pronator quadratus and pronator teres are two examples.
By action
The pronator moves to face downward, the supinator moves to face upward, the abductor moves away from the midline, the adductor moves towards the midline, the depressor moves downward, the elevator moves upward, the flexor moves that decreases an angle, the extensor moves that increase an angle or straightens, internal rotator rotating towards the body, external rotator rotating away from the body; Fixator muscles help to set a joint in a certain position by stabilizing the prime mover while other joints move. Sphincters reduce size, whereas tensors provide tension.
By the number of heads
quadriceps four; triceps three; and biceps two.
By location
called after the nearby major structures, such as the temporal bone and the temporal muscle (temporalis). Additionally, sub-under, infra-below, and supra-above
By fascicle orientation
Rectus, transverse, and oblique all denote orientation concerning the midline: parallel, perpendicular, and diagonal, respectively. with relation to the axis of force generation: pennate and parallel muscle types.
Fiber types
Muscle fiber may be divided into two general categories: Sluggish Type I and Type II, which is quick. Three primary fiber types are found in type II, which is divided into two divisions: type IIA (oxidative) and type IIX (glycolytic).
The metabolic, contractile, and motor unit characteristics of these fibers are comparatively different. These distinct kinds of qualities are shown in the table below.
These kinds of characteristics are often examined at the motor unit level rather than the individual fiber level, even though they are somewhat reliant on the characteristics of individual fibers.
Type I slow oxidative fibers contract more slowly than other types of fibers and generate ATP through aerobic respiration. Fast oxidative (type IIA) fibers can exhaust more quickly than slow oxidative fibers because they can transition to anaerobic respiration (glycolysis) and have rapid contractions.
Anaerobic glycolysis is the primary energy source for rapid glycolytic (type IIX) fibers, which contract quickly. Compared to the other fibers, the FG fibers wear out faster. All three kinds are present in the majority of human skeletal muscles but in different proportions.
Fiber color
In the past, fibers were classified based on the different colors they exhibited, which indicated the amount of myoglobin they contained. Because type I fibers have a high myoglobin content, they appear red. Red muscle fibers often have higher local capillary densities and more mitochondria.
Because they produce ATP through oxidative metabolism, these fibers are more suited for endurance and are slower to tire. Because they rely on glycolytic enzymes and comparatively low myoglobin levels, less oxidative Type II fibers are white.
Twitch Speed
Fibers can also be divided into two categories based on how quickly or slowly they twitch. These characteristics mostly, though not entirely, match the color, ATPase, or MHC-based categories.
Fast twitch fibers, according to some writers, are those in which the myosin can divide ATP very quickly. These mostly consist of MHC type II fibers and ATPase type II fibers.
Fast twitch fibers can, however, also exhibit increased action potential electrochemical transmission capabilities as well as rapid calcium release and sarcoplasmic reticulum absorption.
Fast twitch fibers may contract and create tension at a pace that is two to three times faster than slow twitch fibers. They do this by using a well-developed, anaerobic, short-term glycolytic system. Because they can produce brief bursts of strength or speed more effectively than slow muscles, fast twitch muscles tire out faster.
The slow twitch fibers use a long-term aerobic energy transfer pathway to provide energy for ATP resynthesis. The MHC type I fibers and ATPase type I are the two prominent examples of these.
They frequently exhibit poor ATPase activity, slower contraction rates, and less developed glycolytic capacities. Slow-twitch fibers have more capillaries and mitochondria, which makes them more suitable for continuous work.
Type Distribution
Although different fiber types are often mixed in individual muscles, the quantities of each type change depending on the function of the muscle. In humans, the soleus muscle has around 80% type I fibers, whereas the quadriceps muscles have about 52% type I fibers.
Just around 15% of the eye’s orbicularis oculi muscle is type I. On the other hand, there is little difference in muscle motor units between their fibers. Because of this, the size principle of motor unit recruitment is still applicable.
It has long been believed that the total number of skeletal muscle fibers remains constant. Although the distribution of fiber is thought to be unaffected by sex or age, the proportions of different kinds of fiber change significantly between muscles and individuals. The ratios of different muscle fiber types vary greatly throughout animals.
Men and women who are sedentary have 45% type II and 55% type I fibers, as do small children.[Reference required] Athletes that excel in endurance sports, for example, typically have patterns of fiber distribution, with a larger concentration of type I fibers. However, a lot of types of IIX fibers are needed by sprint competitors.
Athletes competing in middle-distance events exhibit almost equal distributions of the two kinds. This frequently holds for strong athletes like throwers and jumpers as well. There have been suggestions that different forms of exercise can alter a skeletal muscle’s fiber composition.
It is believed that certain type IIX fibers will change into type IIA fibers if endurance-type activities are performed over an extended period. On the other hand, opinions differ on the matter.
It’s possible that following high-intensity endurance training, type IIX fibers exhibit improvements in their oxidative capability, enabling them to carry out oxidative metabolism on par with untrained persons’ slow-twitch fibers.
Not a change in fiber type, but rather an increase in mitochondrial size and quantity and the concomitant alterations that go along with it would cause this.
Fiber typing methods
There are several different techniques used in fiber-typing, and non-experts frequently become confused amongst them. Histochemical staining for myosin ATPase activity and immunohistochemical staining for myosin heavy chain (MHC) type are two techniques that are frequently misunderstood.
The term “fiber type” is routinely and accurately used to describe myosin ATPase activity, which is obtained by the direct assaying of ATPase activity under different circumstances, such as pH.
The most correct word for myosin heavy chain staining is “MHC fiber type,” such as “MHC IIa fibers,” and it comes from identifying the various MHC isoforms. The fundamental determinant of ATPase activity is the MHC type, which makes both approaches physiologically closely linked.
However, neither of these typing techniques directly addresses the fiber’s oxidative or glycolytic capability; rather, they are not metabolic.
In general, whether “type I” or “type II” fibers are mentioned, the term most properly refers to the total of the numerical fiber types (I vs. II) as determined by myosin ATPase activity staining.
The link between these two approaches, restricted to human fiber types, is displayed in the table below. In contrast to MHC typing, subtype capitalization is employed in fiber typing, and several ATPase types indeed include more than one MHC type.
Furthermore, no technique produces expression of subtype B or b in people. ATPase classified humans as IIB because early researchers thought that humans expressed an MHC IIb. Subsequent studies, however, revealed that the human MHC IIb was IIX, suggesting that the IIB is more appropriately called IIX.
Since IIb is expressed in other animals, it is nonetheless correctly reported in the literature alongside IIB. Types of non-human fibres include real IIb fibres, IIc,
IId, and so on.
There is a greater range of less strictly defined ways for further fiber typing. They frequently place more emphasis on functional and metabolic capabilities. As previously mentioned, these properties are not directly measured nor determined by fiber type by ATPase or MHC.
On the other hand, several of the different approaches are associated in vivo, and many of them are linked mechanistically.
For example, the kind of ATPase fiber influences contraction speed because a high level of activity permits quicker cross-bridge cycling.
Type I fibers are “slow” in part because of their lower ATPase activity speeds than Type II fibers, even though ATPase activity is only one factor in contraction speed. Contraction speed measurement is not the same as ATPase fiber typing, however.
Muscle fiber type evolution
For mobility, almost all multicellular creatures rely on their muscles. Both slow- and fast-twitch muscle fibers are typically found in the muscular systems of most multicellular animals, while the relative amounts of each kind of fiber might differ between species and circumstances.
When organisms are placed in changing environments that require either long duration of movement (higher slow twitch proportion) or short explosive movements (higher fast twitch proportion) to survive, their ability to shift their phenotypic fiber type proportions through training and responding to the environment has served them well.
Changes in force output and muscle mass can occur within months, as bodybuilding has demonstrated. Below are some instances of this variant.
Examples of muscle fiber variation in different animals
Invertebrates
Homarus americanus, the American lobster, possesses three different types of fibers: slow-twitch, slow-twitch, and slow-tonic fibers. A slow-tonic fiber has a prolonged contraction time (tonic).
The proportions of the various muscle fiber types in a lobster’s body change according to the function of that particular muscle group.
Vertebrates
During the initial stages of vertebrate embryogenesis, muscle growth, and creation occur in sequential waves or phases. One important factor that determines the particular fiber type is the myosin heavy chain isotype.
Slow-twitch muscle fibers are the first to develop in zebrafish embryos. The aforementioned cells will migrate from their initial site to generate a monolayer of slow-twitch muscle fibers. As the embryo develops, these muscle fibers continue to differentiate.
Reptiles
Larger animals’ various muscle groups will progressively need varying muscle fiber types in varied quantities for various functions. The complementary muscles in the necks of turtles, like Trachemys scripta elegans, may exhibit an inverted trend in the percentages of different fiber types. Turtles’ complimentary muscles had comparable fiber-type percentages.
Mammals
The maximal dynamic force and power production of chimpanzee muscles is 1.35 times greater than that of human muscles of comparable size, with 67% of the muscles being made up of fast-twitch fibers.
Type II fibers are more common in animals and use glycolytic metabolism. Chimpanzees do better in power-related tasks than humans due to differences in fast-twitch fibers. However, humans are better at aerobic activity that requires high metabolic expenses, such as walking.
Genetic conservation versus functional conservation
While certain gene sequences have survived across species, their functions are not necessarily the same. The Prdm1 gene inhibits the development of new slow-twitch fibers in the zebrafish embryo by both direct and indirect means, including Sox6 (indirect).
Although Prdm1 is present in mice, Sox6 does not use it to regulate the genes associated with sluggish muscle in mice.
Plasticity
The mix of muscle fiber types is variable and can change depending on a variety of environmental conditions in addition to having a hereditary foundation. Among creatures with muscle, this flexibility may be the greatest evolutionary benefit.
Different fiber types manifest themselves differently in fish depending on the water temperature. Muscle metabolism must be more effective in colder temperatures, and fatigue resistance is crucial. Fast, strong motions, however, could ultimately be more advantageous in more tropical settings.
It’s common for rodents like rats to have musculature that is unstable. They have up to 60% fast-to-slow changing muscle and a significant number of hybrid muscular fibers.
The amounts of different forms of fiber in people are greatly influenced by environmental factors, including nutrition, activity, and lifestyle choices. The proportions will change towards slow-twitch fibers with aerobic activity, and towards fast-twitch fibers with explosive powerlifting and sprinting.
“Exercise training” in animals will more closely resemble the necessity for extended periods of movement or brief, powerful movements to elude predators or capture prey.
Microanatomy
Under a microscope, skeletal muscle has a characteristic banding pattern because of the organization of two contractile proteins, actin, and myosin, which are two of the myofilaments in the myofibrils.
The thick filaments, which are organized into repeating units called sarcomeres, are made of myosin, and the thin filaments are made of actin. Muscle contraction is the result of both proteins interacting.
The cytoskeleton’s intermediate filaments allow the sarcomere to be connected to other organelles like the mitochondria. The sarcomere and sarcolemma are joined by the costamere.
A muscle fiber’s organelles and macromolecules are all ordered to fulfill certain roles. The cytoplasm is referred to as sarcoplasm, while the cell membrane is referred to as the sarcolemma. The myofibrils are located in the sarcoplasm.
The lengthy protein bundles known as myofibrils have a diameter of one micrometer. The peculiarly flattened myonuclei are pressed up against the inside of the sarcolemma. The mitochondria are found in between the myofibrils.
The muscle fiber has a sarcoplasmic reticulum but lacks smooth endoplasmic cisternae. Encircling the myofibrils, the sarcoplasmic reticulum stores a supply of calcium ions that are required for muscle contraction.
It periodically possesses terminal cisternae, which are dilated end sacs. These go from one side of the muscle fiber to the other. A transverse tubule is a tubular infolding located between two terminal cisternae.
Action potentials travel via T tubules to instruct the sarcoplasmic reticulum to release calcium, which results in a contraction of the muscle.
A transverse tubule, two terminal cisternae, and one other form a trio.
Development
Myoblasts, or muscle progenitor cells, either move out into the body to generate all other muscles during development, or they stay in the somite to form muscles connected to the spinal column.
The creation of connective tissue frameworks, which are typically derived from the somatic lateral plate mesoderm, occurs before myoblast migration. Myoblasts are directed to the proper sites by chemical cues, where they unite to form elongated, multinucleated skeletal muscle cells.
All muscle cells have fast myosin-heavy chains between weeks ten and eighteen of pregnancy; in the growing fetus, two kinds of myotubes become identifiable, one expressing both fast and slow chains and the other expressing just fast chains.
Ten to forty percent of the fibers have delayed myosin chain expression. The paraxial mesoderm is the source of all muscles. Somatyogenesis is the process by which the paraxial mesoderm divides down the length of the embryo to generate somites.
This process corresponds to the body segmentation that is most clearly observed in the vertebral column during embryonic development.
Three divisions occur in each somite: the sclerotome, which forms the vertebrae, the dermatome, which develops the skin, and the myotome, which forms the muscle.
The epimere and hypomere, which make up the epaxial and hypaxial muscles, respectively, are the two portions of the myotome.
The dorsal rami of the spinal nerves innervates the erector spinae and tiny vertebral muscles, which are the only epaxial muscles in humans. All other muscles, including those in the limbs, are innervated by the spinal nerves’ ventral rami and are hypaxial.
During embryonic development, fiber types are determined, and later in adulthood, effects from the nervous and hormonal systems modify them. For the postnatal development of muscle cells, the number of satellite cells under the basal lamina is essential.
Function
Muscle contraction is its main purpose. Skeletal muscle secretes myokines—a variety of cytokines and other peptides that serve as signaling molecules—after contraction, acting as an endocrine organ. It is therefore thought that myokines modulate the health advantages of exercise.
Following a muscular contraction, myokines are released into circulation. The most researched myokine is interleukin 6 (IL-6); additional myokines generated by muscular contraction include BDNF, FGF21, and SPARC.
In addition, muscle generates body heat. The contraction of muscles produces eighty-five percent of the heat in the body. This heat is mainly squandered and is created as a byproduct of muscle action. To produce heat, muscles are signaled to contract in a homeostatic manner in reaction to excessive cold.
Contraction
The muscle fiber, which is its structural unit, and the motor unit, which is its functional unit, work together to produce a contraction. Motor neurons excite the excitable cells that makeup muscle fibers.
A motor neuron and the numerous fibers it comes into touch with make up the motor unit. Numerous motor units can excite a single muscle. At the neuromuscular junctions, motor neurons produce the neurotransmitter acetylcholine, which depolarizes muscle fibers.
Troponin and tropomyosin are two additional critical regulatory proteins that enable muscle contraction in addition to the actin and myosin myofilaments in the myofibrils that comprise the contractile sarcomeres. Actin and these proteins work together to stop actin from interacting with myosin.
Ionic calcium (Ca2+) is released by the sarcoplasmic reticulum of a suitably activated cell, and it subsequently interacts with the regulating protein troponin.
Troponin bound to calcium changes shape, causing tropomyosin to migrate and actin’s myosin-binding sites to become visible. This permits the muscle to shorten and undergo ATP-dependent cross-bridge cycling involving myosin and actin.
Excitation-contraction coupling
The mechanism by which a muscular action potential in the muscle fiber induces the myofibrils to contract is known as excitation-contraction coupling.
This mechanism is based on a direct link between voltage-gated L-type calcium channels and the sarcoplasmic reticulum calcium release channel RYR1.
The RyRs live across the SR membrane, but the DHPRs are found on the sarcolemma, which includes the transverse tubules and the surface sarcolemma. Known as a trio, excitation–contraction coupling mostly occurs in the proximity of a transverse tubule and two SR areas containing RyRs.
Excitation-contraction coupling happens when skeletal muscle cell depolarization produces a muscle action potential. This potential then depolarizes the inner part of the muscle fiber by propagating past the cell surface and into the network of T-tubules within the muscle fiber.
Dihydropyridine receptors in the terminal cisternae, which are near ryanodine receptors in the nearby sarcoplasmic reticulum, are activated by depolarization of the inner sections. The physics dihydropyridine receptors are active.
A calcium flash is produced when the sarcoplasmic reticulum releases Ca2+ into the immediate junctional space and diffuses into the bulk cytoplasm when the ryanodine receptors open.
The large calcium buffering capability of the sarcoplasmic reticulum is partly attributed to a calcium-binding protein known as calsequestrin.
The action potential activates hundreds of calcium sparks almost simultaneously, increasing calcium levels throughout the cell and resulting in the upstroke of the calcium transient.
Actin filaments bind Troponin C when Ca2+ is released into the cytosol, enabling crossbridge cycling and, in certain cases, motility. The active pumping of Ca2+ back into the sarcoplasmic reticulum is carried out by the sarco/endoplasmic reticulum calcium-ATPase (SERCA). The force decreases and relaxation takes place when Ca2+ returns to resting levels.
Muscle movement
The peripheral nervous system’s efferent leg is ultimately in charge of directing orders to the muscles and glands, which results in voluntary movement. Both voluntary and autonomic (involuntary) impulses from the brain cause nerves to contract muscles.
Just in front of the central sulcus that separates the frontal and parietal lobes, certain areas in the primary motor cortex of the brain correspond to deep, superficial, facial, and internal muscles.
Furthermore, muscles respond to reflexive nerve stimulation, which does not necessarily transmit messages to the brain. In this instance, the afferent fiber’s signal is directly connected to the efferent nerves in the spine to cause reflexive movement rather than reaching the brain.
But most muscle movement is voluntary, the product of intricate connections between different brain regions.
Mammal skeletal muscle control nerves line up with neuronal groupings along the cerebral cortex’s main motor cortex. Before commands are transmitted by the pyramidal tract to the spinal cord and then to the motor end plate at the muscles, they first pass via the basal ganglia and are subsequently changed by information from the cerebellum. Along the way, inputs from feedback systems like the extrapyramidal system affect muscle tone and reaction.
Deeper muscles, such as those involved in posture, are frequently regulated by basal ganglia and brain stem nuclei.
Proprioception
Muscle spindles in skeletal muscles communicate to the central nervous system the degree of muscle length and stretch, which helps to maintain joint position and posture.
Proprioception is the sensation of where our bodies are in space; it is the “unconscious” awareness of where the different parts of the body are at any given moment in time.
Utilizing feedback from proprioception, many brain regions synchronize movement and orientation. To ensure smooth motion, the cerebellum and red nucleus in particular constantly sample position against movement and make small adjustments.
Energy consumption
The majority of the energy used by the body comes from muscular action. Adenosine triphosphate (ATP) molecules are produced by all muscle cells and are responsible for the myosin heads’ motion.
Created from ATP, creatine phosphate serves as the muscles’ short-term energy reserve. When needed, creatine kinase may renew ATP. Glycogen, a kind of stored glucose, is also maintained in muscles. For strong, prolonged contractions, glucose can be quickly transformed from glycogen.
The glucose molecule can be metabolized anaerobically in the voluntary skeletal muscles through a process known as glycolysis, which yields two ATP and two lactic acid molecules.
Globules of fat are also found in muscle cells, and they are utilized as an energy source during aerobic activity. Anaerobic glycolysis yields far less ATP than aerobic energy sources, which need many more metabolic processes and take longer to attain optimal efficiency.
On the other hand, cardiac muscle always extracts the greatest amount of ATP yield from any molecule involved and can easily absorb any of the three macronutrients (protein, glucose, and fat) aerobically without the need for a “warm-up” time. During exercise, skeletal muscle produces and excretes lactic acid, which is also consumed by the heart, liver, and red blood cells.
Compared to other organs, skeletal muscle needs more calories. It uses 54.4 kJ/kg each day while at rest. This is more than bone, which is 9.6 kJ/kg, and adipose tissue (fat), which is 18.8 kJ/kg (4.5 kcal/kg).
Efficiency
Human muscular efficiency has been calculated to be between 18% and 26% when it comes to cycling and rowing. Oxygen consumption may be used to determine efficiency, which is defined as the ratio of mechanical effort production to total metabolic cost.
This poor efficiency is caused by losses in the conversion of energy from ATP into mechanical work inside the muscle, mechanical losses inside the body, and an approximately 40% efficiency in creating ATP from dietary energy.
The type of exercise and the muscle fibers being utilized (fast- or slow-twitch) determine whether the latter two losses occur. One watt of mechanical power is equal to 4.3 kcal per hour with an overall efficiency of 20 percent.
One producer of rowing apparatus, for instance, sets up its rowing ergometer to measure burnt calories as four times the actual amount of mechanical labor + 300 kcal per hour. This is around 20% efficiency at 250 watts of mechanical output.
Numerous factors can influence the mechanical energy production of a cyclic contraction, such as the time of activation, the trajectory of muscle strain, and the rates of force increase and decay. Work loop analysis can be used to synthesize these experimentally.
Muscle strength
- Grade 0 No contraction
- Grade 1: There is a trace of contraction but no joint movement
- Grade 2: Gravity-free movement at the joint
- Grade 3: Motion defying gravity but not defying additional resistance
- Grade 4: Less than usual movement against external resistance
- Grade 5: Average power
Three overlapping factors determine muscle strength: neurological strength (the strength of the signal that instructs a muscle to contract), mechanical strength, and physiological strength.
When isometric and at maximum length, vertebrate muscle normally generates 25–33 N (5.6–7.4 lbs) of force per square centimeter of muscle cross-sectional area. Certain invertebrate muscles, like the claws on crabs, have sarcomeres that are far longer than those of vertebrates. This means that there are many more locations for actin and myosin to bind, which increases force per square centimeter but decreases speed significantly.
Measuring the force produced by a contraction can be done in vivo using tendon strain, non-invasively using phonomyography or mechanomyography, or directly using more invasive techniques.
Any particular muscle’s strength, measured in terms of force applied to the skeleton, is determined by its length, myosin isoforms, cross-sectional area, pennation, sarcomere length, and neural activation of its motor units. Serious declines in muscular strength may point to underlying disease; the chart on the right might be used as a reference.
According to Rohmert’s law, the maximal holding period of a contracted muscle is contingent upon its energy supply and decreases exponentially with the start of the effort.
The “strongest” human muscle
- It is false to compare the strength of individual muscles and declare one to be the “strongest” as three elements influence muscular strength at the same time and muscles never function independently. However, there are a few muscles below whose strength is notable for various reasons.
- Muscular “strength” is commonly used to describe the capacity to apply force to an external object, such as lifting a weight. The masseter, or jaw muscle, is the strongest by definition. The accomplishment of a biting strength of 4,337 N (975 lbs) for two seconds is documented in the 1992 Guinness Book of Records.
- The masseter is unique among muscles because of its ability to act against a somewhat shorter lever arm than other muscles. This is what sets it apart from other muscles.
- The strongest muscles are those with the highest cross-sectional area if “strength” is defined as the force applied by the muscle itself, such as at the point where it enters into a bone. This is due to the relatively constant stress that each skeletal muscle fiber exerts.
- The maximum force that each fiber can produce is 0.3 micronewtons. According to this description, the quadriceps femoris or gluteus maximus are typically cited as the strongest muscles in the body.
- A shorter muscle will be stronger “pound for pound” (i.e., by weight) than a longer muscle of the same cross-sectional area since muscular strength is dictated by cross-sectional area. The uterine myometrial layer may be the strongest muscle in a woman’s body in terms of weight.
- The total weight of the uterus during delivery is around 1.1 kg (40 oz). The uterus contracts between 100 and 400 N (25 and 100 lbs) of downward force each time a woman gives birth.
- The incredibly large and strong external muscles of the eye dwarf the size and weight of the eyeball. According to popular belief, they are “the strongest muscles for the job they have to do” and, on rare occasions, “100 times stronger than they need to be.”
- rapid movement of the eyes Every night while you sleep, your eye muscles become exercised; still, rapid eye movements do require rapid movements.
- The assertion that “the tongue is the strongest muscle in the body” is frequently seen in lists of surprising facts, despite the difficulty in finding a definition of “strength” that would support this claim. The tongue is made up of eight muscles, not just one.
Force generation
Muscle velocity is proportionate to muscle fiber length, and muscle force is related to physiological cross-sectional area (PCSA).
However, a variety of biomechanical factors, such as the spacing between muscle insertions and pivot points, the size of the muscle, and the architectural gear ratio, affect the torque around a joint.
Normally, muscles are positioned in opposition to one another, such that while one group contracts, the other group lengthens or relaxes.
It is not feasible to completely trigger the contraction of two antagonistic muscles at the same time due to antagonism in the transmission of nerve impulses to the muscles.
The antagonist’s muscles function as a “brake” for the agonist’s muscles during contraction, especially towards the finish of a ballistic motion like throwing
While the muscles in the back and rear of the shoulder engage and experience eccentric contraction to slow the motion down to prevent damage, the chest and front of the shoulder contract to bring the arm forward in the throwing example.
Learning to release the antagonist muscles to enhance the anterior shoulder and chest force input is part of the training process.
Muscle contraction results in sound and vibration. Ten to thirty contractions per second (10 to 30 Hz) are produced by slow twitch fibers.
Thirty to seventy contractions per second are produced by fast twitch fibers (30 to 70 Hz). You may see and feel the vibration by tensing your muscles to the point of producing a tight fist.
Again, a tight fist is an excellent illustration of how to push a strongly tensed muscle against the ear to hear the sound. Most descriptions of the sound characterize it as rumbling.
Some people may deliberately create this rumbling sound by tensing their middle ear’s tensor tympani muscle. If the muscles in the jaw or neck are stiff, you might also hear a rumbling sound.
Signal transduction pathways
In mature animals, the phenotype of skeletal muscle fibers is controlled by many distinct signaling pathways. These pathways include those involving calcineurin, calcium/calmodulin-dependent protein kinase IV, the Ras/mitogen-activated protein kinase (MAPK) pathway, and peroxisome proliferation.
coactivator 1. By linking excitation and transcription control, the Ras/MAPK signaling pathway connects motor neurons to signaling systems and facilitates the nerve-dependent induction of the slow program in regenerated muscle.
The transcription factor NFAT’s phosphorylation state is directly regulated by calcineurin, a Ca2+/calmodulin-activated phosphatase that is involved in nerve activity-dependent fiber-type specification in skeletal muscle.
This permits NFAT’s translocation to the nucleus and activates slow-type muscle proteins in conjunction with myocyte enhancer factor 2 (MEF2) proteins and other regulatory proteins.
Slow motor neuron activity also upregulates Ca2+/calmodulin-dependent protein kinase activity, presumably because it magnifies the responses generated by slow-type calcineurin by enhancing oxidative capacity through stimulation of mitochondrial biogenesis and by promoting MEF2 transactivator functions.
Changes in intracellular calcium or reactive oxygen species brought on by contraction send signals to a variety of pathways, such as calcineurin, MAPKs, and calcium/calmodulin-dependent protein kinase IV, which in turn activate transcription factors that control skeletal muscle gene expression and enzyme activity.
Selective slow twitch (ST) muscle genes are synergistically activated by PGC1-a (PPARGC1A), a transcriptional coactivator of nuclear receptors crucial for the regulation of several mitochondrial genes involved in oxidative metabolism. PGC1-a (PPARGC1A) also acts as a target for calcineurin signaling.
Skeletal muscle fiber phenotypic modulation is controlled via a transcriptional pathway mediated by peroxisome proliferator-activated receptor d (PPARd). Mice with an active version of PPARd exhibit an “endurance” phenotype, characterized by a corresponding increase in mitochondrial biogenesis and oxidative enzymes, as well as a higher percentage of ST fibers.
Thus, via functional genomics, calcineurin, PGC-1a, calmodulin-dependent kinase, and activated PPARd constitute the building blocks of a signaling network that regulates the change of skeletal muscle fiber type and metabolic profiles that guard against obesity and insulin resistance.
Several processes must be quickly triggered during intensive exertion to guarantee a steady supply of ATP for the working muscles when the body switches from aerobic to anaerobic metabolism.
These include a shift in blood flow from non-working to active muscles, a conversion from fat-based to carbohydrate-based fuels, and the elimination of lactic acid and carbon dioxide, two byproducts of anaerobic metabolism.
The transcriptional regulation of the fast twitch (FT) glycolytic phenotype controls some of these responses.
For example, the Six1/Eya1 complex, which is made up of proteins from the Six protein family, is involved in the reprogramming of skeletal muscle from an ST glycolytic phenotype to an FT glycolytic phenotype.
Furthermore, a master regulator for the production of genes involved in critical hypoxic responses that preserve ATP levels in cells is the hypoxia-inducible factor 1-a (HIF1A). In skeletal muscle, HIF-1a ablation was linked to an increase in the activity of mitochondrial rate-limiting enzymes.
This suggests that the animals’ enhanced fatty acid oxidation and citric acid cycle may be making up for the lower flow through the glycolytic route. Nonetheless, the control of mitochondrial dysfunction is also associated with hypoxia-mediated HIF-1a responses, since they lead to the creation of excessive reactive oxygen species inside the mitochondria.
Adult muscle character is also influenced by other mechanisms. For instance, applying physical stress within a muscle fiber can change muscle development by releasing the transcription factor serum response factor from the structural protein titin.
Clinical significance
Muscle disease
Myopathies are disorders of the skeletal muscle, whereas neuropathies are disorders of the nerves. Both are classified as neuromuscular diseases and can impair muscle function or result in discomfort in the muscles.
Mutations in the different related muscle proteins are thought to be the root cause of many myopathies. Inflammatory myopathies include polymyositis and inclusion body myositis.
Muscular dystrophy causes the afflicted tissues to become disorganized and significantly lowers the content of dystrophin (green).
Neuromuscular disorders impact the neurological system that controls the muscles. In general, depending on the location and kind of the issue, nerve control issues can result in either spasticity or paralysis.
Many movement abnormalities are brought on by neurological conditions involving central nervous system malfunction, such as Parkinson’s disease and Huntington’s disease.
Muscle illnesses can cause myalgia, weakness, spasticity, and myoclonus. Electromyography, which measures the electrical activity in muscles, and blood tests for creatine kinase levels are two diagnostic techniques that may show diseases related to the muscles.
In rare circumstances, a muscle biopsy may be performed to diagnose a myopathy, and genetic testing may be used to detect anomalies in DNA linked to certain myopathies and dystrophies.
To monitor neuromuscular illness, a non-invasive elastography technology measuring muscle noise is being experimented with. The actomyosin filaments shortening along the muscle’s axis generates a muscle’s sound. The muscle contracts by shortening its length and widening its breadth, which causes surface vibrations.
Numerous variables, including hormone signaling, developmental factors, strength training, and illness, can cause muscles to grow bigger, independent of strength and performance assessments. Exercise cannot increase the number of muscle fibers, despite what the general public believes.
Instead, when new protein filaments are added, muscles become bigger as a result of both muscle cell development and the mass that undifferentiated satellite cells add to the existing muscle cells.
Age and hormone levels are two biological elements that might impact muscle hypertrophy. In boys going through puberty, the body produces more growth-stimulating hormones, which causes hypertrophy to occur more quickly.
Given that testosterone is one of the body’s primary growth hormones, males often have a far easier time achieving hypertrophy than women. Increasing your testosterone or other anabolic steroid intake will cause your muscles to become larger.
Building muscle is influenced by neurological, spinal, and muscular processes. Even when just the opposite muscle has been worked, a person may occasionally detect an improvement in strength in a particular muscle.
For example, a bodybuilder may discover that her left biceps are stronger after finishing a program that exclusively worked her right biceps. We refer to this phenomenon as cross-education.
Atrophy
One to two percent of muscle is broken down and replaced every day. Sarcopenia or muscular atrophy can result from a breakdown that is accelerated by aging, illness, inactivity, and starvation. Age-related sarcopenia is frequently associated with frailty and its aftereffects. A drop in protein content and a reduction in the size and quantity of muscle cells may occur along with a loss of muscle mass.
It is known that extended durations of weightlessness and immobilization during human spaceflight cause muscle atrophy and weakening, which can cause some muscles to lose up to 30% of their mass. Some animals have also been seen to have the same effects after hibernating.
Cachexia is the term for the loss of muscle caused by a variety of illnesses and ailments, such as cancer, AIDS, and heart failure.
Research
Muscle cell culture systems from healthy or diseased tissue biopsies have been used to replicate myopathies. The guided development of pluripotent stem cells provides another supply of skeletal muscle and progenitors.
Skeletal muscle characteristics are studied using a variety of methods. Utilizing electrical muscle stimulation, one may ascertain the power and speed of contraction at various frequencies associated with the mix and composition of fiber types inside a certain muscle area. For a more thorough description of muscle characteristics, in vitro muscle testing is utilized.
Electromyography (EMG) measures the electrical activity related to muscle contraction. There are two physiological reactions in skeletal muscle: contraction and relaxation. The electrical activity detected by EMG is produced by the processes behind these reactions.
EMG is specifically capable of measuring a skeletal muscle’s action potential, which is caused by the motor axons’ hyperpolarization in response to nerve impulses that are sent to the muscle. In studies, EMG is used to measure the force produced, identify signs of muscular exhaustion, and ascertain if the target skeletal muscle is being engaged.
Intramuscular EMG and surface EMG, which is the most frequent form, are the two types of EMG. When a skeletal muscle contracts as opposed to relaxes, the EMG signals are substantially stronger. Nevertheless, the EMG signals for deeper and smaller skeletal muscles
Mononuclear cells of skeletal muscle
About half of the nuclei in skeletal muscle are those of myocytes and the other half are those of mononuclear cells. Mononuclear cells discovered in human and mouse skeletal muscle samplesCell type indicators encoded in messenger RNA can be used to identify. Nine cell types were discovered by Cameron et al.
These comprise fibro-adipogenic progenitors (FAPs) (20%), endothelial cells that line capillaries (45% of cells), pericytes (14%) and endothelium-like pericytes (4%). Muscle stem cells make up an additional 9% of mononuclear cells that are found next to muscle fiber cells.
Most of the remaining mononuclear cells in skeletal muscle were composed of myeloid cells, such as macrophages, which made up 2%, and lymphoid cells, such as B-cells and T-cells, which made up 3%.
Cameron et al. also distinguished between Type I and Type II myocyte cells. It has been discovered that distinct gene sets are expressed by the various cell types that make up skeletal muscle. 1,331 genes were the median number of genes expressed in each of the nine distinct cell types.
On the other hand, a biopsy obtained from a thigh muscle includes every sort of cell. A biopsy of the skeletal muscle of the human thigh revealed 13,026–13,108 genes that were found to be expressed.
Endocrine functions of skeletal muscle
As mentioned in the article’s Introduction, the secretome of skeletal muscles contains subsets of 654 distinct proteins as well as lipids, amino acids, metabolites, and short RNAs under various physiological circumstances.
Skeletal muscle is classified as an endocrine organ since it secretes cytokines and other peptides that serve as signaling molecules, as stated in the Wikipedia page “List of human endocrine organs and actions”.
Skeletal muscle “synthesizes and secretes multiple factors, and these factors exert beneficial effects on peripheral and remote organs,” according to Iizuka et al., which indicates that skeletal muscle is an endocrine organ. The secretomes of inactive muscle and those changed by resistance or endurance exercise appear to exert a wide range of impacts on distant tissues.
Sedentary skeletal muscle mass affects executive mental function
A Canadian study examined the impact of muscle mass on cognitive abilities as people age. The study hypothesized that the skeletal muscle-specific secretome’s endocrine components would shield cognitive processes. 8,279 Canadians over 65 who were in average health had their skeletal muscle mass evaluated at baseline and three years later in their arms and legs.
Less than 7.30 kg/m2 for men and less than 5.42 kg/m2 for women were the low skeletal muscle mass thresholds for 1,605 participants (19.4%) of these people at baseline.
Measurements were taken of psychomotor speed, memory, and executive mental function both at baseline and three years later. Standard tests, such as the Stroop test and the capacity to name several animals in a minute or the sequence 1-A, 2-B, 3-C, were used to evaluate executive mental function.
According to the study, the deterioration in executive mental function was noticeably more severe in those with lower skeletal muscle mass at the beginning of the trial than in those with higher muscle mass.
Conversely, there was no relationship between skeletal muscle mass and psychomotor speed or memory. Therefore, greater muscle mass and a correspondingly larger secretome seem to have an endocrine role that safeguards older adults’ executive mental performance.
Walking, using skeletal muscles, affects mortality
Paluch et al. examined the relationship between the average daily step count and mortality risk in persons 60 and older. The research included a meta-analysis of 15 studies that examined 47,471 people over 7 years.
People were separated into roughly equal groups called quartiles. The average daily step count for the lowest quartile was 3,553, for the second, 5,801; the third, 7,842; and for the fourth, 10,901 steps.
Walking pace, while controlling for walking volume, did not affect death. The daily step count, however, was correlated with death.
The relative risk of mortality for the second, third, and fourth quartiles was 0.56, 0.45, and 0.35, respectively, when the risk of death for individuals over 60 was set as 1.0 for the lowest quartile of steps/day.
The findings weren’t as noticeable for people under 60. When the first quartile risk of death for those under 60 was set as 1.0, the corresponding relative risks of death for the second, third, and fourth quartiles were, respectively, 0.57, 0.42, and 0.53. Consequently, walking’s demand on the skeletal muscles has a significant impact on mortality, particularly for the elderly
Skeletal muscle secretome alters with exercise
Williams et al. collected biopsies of the skeletal muscles in the thighs of eight Caucasian guys, ages 23 who had previously been sedentary.
Biopsies were obtained both before and during a training program consisting of six weeks of endurance exercise. For six weeks, the exercise entailed one hour of stationary cycling five days a week.
Following endurance training, 641 genes showed an increase in expression out of 13,108 genes whose expression was found in the muscle samples, while 176 genes showed a decrease.
Out of all the changed genes (817 total), 531 were shown to be in the secretome by Uniprot, Exocarta, or other research looking at the secretome of muscle cells.
The fact that a large number of the genes regulating exercise are found to be released suggests that a large portion of exercise’s effects are endocrine rather than metabolic.
Exercise-regulated proteins that were released were discovered to primarily impact pathways associated with heart, brain, kidney, and platelet functioning
Exercise-Trained Effects Are Mediated By Epigenetic Mechanisms
At least 25 studies published between 2012 and 2019 suggested that skeletal muscle responses to exercise are significantly influenced by epigenetic processes. The addition of methyl groups to DNA cytosines or removal of methyl groups from DNA cytosines, particularly at CpG sites, are common mechanisms underlying epigenetic modifications.
Cysteine methylations can condense DNA into heterochromatin, preventing other molecules from accessing the DNA. Histone tail acetylations or deacetylations inside chromatin are another common way that epigenetic modifications take place.
DNA in the nucleus is often found in segments of 146 base pairs wrapped around eight densely packed histones in a configuration known as a nucleosome. Linker DNA connects one DNA segment on a nucleosome to an adjacent DNA segment. Acetylation of histone tails often results in the loosening of the DNA around the nucleosome, increasing the DNA’s accessibility.
Exercise-induced regulation of genes in muscles
In other tissues, muscle tissue’s gene expression is mostly controlled by enhancers and other regulatory DNA sequences. Enhancers are non-coding DNA sequences that, via looping around and interacting with the promoters of their far target genes, stimulate the expression of those distant target genes.
Williams et al. revealed that there are 239,000 nucleotide bases on average between the associated enhancers and promoters of genes in a loop.
DNA methylation or demethylation changes brought on by exercise that affect how genes are expressed
By changing the epigenetic DNA methylation or demethylation of CpG sites inside enhancers, endurance muscle exercise modifies the expression of muscle genes.
Twenty-three inactive, about 27-year-old adults consented to do endurance training on just one leg for three months as part of a research by Lindholm et al. The untrained control leg was the other leg. For three months, the training entailed one-legged knee extension exercises.
Before the start of training and 24 hours following the previous training session, skeletal muscle samples from the vastus lateralis were obtained from each leg. There were notable alterations in DNA methylation at 4,919 different locations throughout the genome in the endurance-trained leg as compared to the untrained limb.
Enhancers included the majority of the changed DNA methylation sites.
Using RNA sequencing for transcriptional analysis, 4,076 genes with differential expression were found.
Significantly lower DNA methylation enhancers were linked to transcriptionally upregulated genes, whereas significantly higher DNA methylation enhancers were linked to transcriptionally downregulated genes.
Genes related to glucose metabolism and muscle structural remodeling were mostly linked to increased methylation. Reduced methylation enhancers were linked to genes involved in transcriptional regulation, inflammatory, or immunological processes.
Long-term changes in gene expression brought on by exercise-induced histone acetylation or deacetylation
As shown above, hundreds of muscle genes have their long-term expression changed by epigenetic modifications to enhancers following exercise. This contains genes that produce systemic circulation-secreted proteins, many of which have the potential to function as endocrine messengers.
Before beginning an exercise regimen, six inactive, Caucasian guys, around 23 years of age, performed vastus lateralis biopsies. Many genes had continuously changed epigenetic expression four days following the end of this workout program.
The modifications changed the histone tails’ acetylations and deacetylations in the enhancers governing the genes with changed expression.
Epigenetic acetylations added at histone 3 lysine 27 of nucleosomes situated in their enhancers were linked to up-regulated genes. The elimination of epigenetic acetylations at H3K27 in nucleosomes positioned at their enhancers was linked to down-regulated genes.
Before the exercise training program, 13,108 genes were expressed at baseline in vastus lateralis muscle biopsies. Biopsies of the same muscles taken four days after the exercise program ended revealed changed gene expression, with 176 genes down-regulated and 641 genes up-regulated.
Williams et al. found 599 enhancer-gene interactions including 268 genes and 491 enhancers in which the associated target gene and the enhancer were synchronously up- or down-regulated following exercise training.
Exercise
It is frequently advised to engage in physical activity to enhance joint function, muscle and bone strength, motor abilities, and fitness. Exercise affects bone, connective tissue, muscles, and the nerves that fire the muscles in several ways.
Muscle hypertrophy, or an increase in muscle mass brought on by an increase in the number of muscle fibers or myofibril cross-sectional area, is one such result. The type of exercise employed determines the changes in muscles.
Aerobic and anaerobic workout regimens are the two main categories. Marathons and other low-intensity, long-duration exercises need the muscles to contract at a strength below their maximum during aerobic activity.
To get metabolic energy for aerobic activities, fat, protein, carbs, and oxygen must be used by aerobic respiration.
A larger proportion of Type I muscle fibers, which are predominantly made up of oxidation and mitochondrial enzymes related to aerobic respiration, is found in the muscles used in aerobic activity.
Anaerobic exercise, on the other hand, is linked to quick, high-intensity exercises like weightlifting or running. Type II fast-twitch muscle fibers are primarily used in anaerobic exercises. During anaerobic activity, type II muscle fibers use glucogenesis as their energy source.
Type II fibers are fatigable, create a lot of lactic acids, and use little oxygen, protein, or fat during anaerobic activity. A lot of workouts, including rock climbing and football, combine anaerobic and aerobic phases.
The production of ATP in muscles is inhibited by the presence of lactic acid. If the intracellular concentration rises too high, it might potentially put an end to the synthesis of ATP. However because endurance exercise increases myoglobin and capillarization, it lessens the accumulation of lactic acid.
This improves the muscles’ capacity to expel waste materials, such as lactic acid, without compromising muscular function. After leaving the muscles, lactic acid is either sent to the liver where it is transformed back into pyruvate, or it can be utilized as an energy source by other muscles or other tissues.
Hard activity causes the muscles to lose potassium ions in addition to raising the lactic acid content. Preventing exhaustion may help muscles recuperate and function again.
Pain or discomfort that appears one to three days after exercise and usually goes away two to three days later is known as delayed onset muscle soreness.
A more recent idea suggests that it is produced by microscopic rips in the muscle fibers brought on by eccentric contraction, or by exercising at levels that are not acclimated to the body’s lactic acid build-up. Days after exercise, the discomfort could not be explained by lactic acid dispersing quickly.
Conclusion
Skeletal muscle in humans is frequently described as a mechanical apparatus that produces force, movement, and contraction. A comprehensive molecular map of the skeletal muscle has started to take shape over the last few decades, with each molecular participant being linked to a certain “functional component” of the muscle.
Even while some of these functional elements—like the NMJ’s sensory apparatus or contractile machinery—have gotten a lot of attention, there hasn’t yet been a thorough analysis of the known molecular elements and how they relate to muscle function.
The objective of this review is to shed light on the molecular components of muscle and the intricate molecular cross-talk that affects and contributes to the function and health of muscle tissue with its many interacting partners.
We recognize that, because of the scale of muscle research, it would be extremely difficult and outside the current purview of this page to fully outline every noteworthy contribution within each of the components presented here.
Nonetheless, the thorough systems-level molecular and functional pathway viewpoints offered in this review aim to acquaint the reader with significant muscle mechanisms that not only open the door to a more in-depth examination of muscle function in health and disease but also offer fascinating insights into the molecular machinery at the center of muscle function.
More study is necessary to understand how skeletal muscle adapts to insults and injuries by trying to return to a precursor-like condition, as evidenced by the increased production of fetal gene isoforms.
The contextual processes outlined here serve as the foundation for additional research on the accuracy and constraints of pharmaceutical treatments.
FAQ
Every organ or muscle is composed of skeletal muscle, connective tissue, nerve tissue, and blood or vascular tissue. There are wide variations in the size, form, and fiber arrangement of skeletal muscles.
Muscle that is subject to conscious control is referred to as skeletal muscle, voluntary muscle, or striated muscle. Skeletal muscles often pass via at least one joint as they travel between bones. Each muscle is composed of tendons, blood vessels, nerves, and muscle tissue. Tendons often connect skeletal muscles to the bone.
Humans can move and carry out daily tasks thanks to their skeletal muscles. They support proper posture and balance and are crucial to respiratory mechanics. They also shield the body’s essential organs.
Skeletal muscles are vital for whole-body metabolism in addition to being required for movement and physical performance. They control blood glucose levels by the insulin-mediated absorption of glucose; if this process is compromised, insulin resistance may result in type 2 diabetes.
In many clinical situations as well as for athletic performance, skeletal muscle mass is essential. The best method for encouraging skeletal muscle growth and remodeling is to combine protein consumption with resistance training. But to be truly effective, certain prerequisites must be met.
use free weights.
use stationary weight machines.
resistance band exercises.
body weight workouts like squats and pushups.
strength training programs that include any or all of the aforementioned exercises.
Numerous studies have demonstrated a positive correlation between high levels of skeletal muscle mass and lean body mass and metabolic health, including high levels of insulin sensitivity. However, a substantial body of research has also demonstrated a negative correlation between high lean body mass and a metabolically unhealthy phenotype.
Skeletal muscle, the most prevalent tissue in the body, has been recognized for more than a century to have the capacity to regenerate new muscle fibers following damage from trauma or illnesses like muscular dystrophy.
Skeletal muscles are the pump muscles used in breathing. The diaphragm muscle, which serves as a ventilatory pump in animals, is the primary inspiratory muscle.
Gaining muscle mass may potentially have an impact on how cardiovascular disease risk is metabolized. Moreover, there exists a correlation between insulin resistance and cardiovascular disease. Since the body uses skeletal muscle mass for 85% of its glucose clearance, having more muscle mass may enhance metabolic health.
Skeletal muscles are located in between the bones throughout your body and around some of the holes in it. The muscle spans a joint, fastens to one end of the bone, and then fastens to another bone. Fibrous connective fibers called tendons help hold your muscles firmly in place on your bones.
The skeletal muscle groups that comprise the upper body include the pectoral, biceps, triceps, latissimus dorsi, trapezius, abdomen, and erector spinae. The gastrocnemius, soleus, gluteus, and quadriceps are the main skeletal muscle groups in the lower body. When muscles contract, they move.
Tendons, which are bands of elastic collagen tissue, connect this muscle to the bones. Connective tissues make up these tendons. The fascicule, a bundle of muscular fibers, makes up the skeletal muscles. The image depicts the cylindrical form of these fascicules.
The striated muscle cell type known as skeletal muscle cells, which are divided into several muscle tissues throughout the body, including the biceps, are what give our muscles the ability to move. Skeletal muscles are typically 2 to 3 cm long, but they can reach up to 30 cm in length thanks to tendons that connect them to the bones.
Your body is thought to include around 650 named skeletal muscles. You can truly have billions of smooth muscle cells since smooth muscle, like other muscle tissue, normally develops at the cellular level. Your body’s muscles carry out several essential tasks.

6 Comments