Emery–Dreifuss Muscular Dystrophy
Table of Contents
What is Emery-Dreifuss muscular dystrophy (EDMD)?
Emery-Dreifuss muscular dystrophy (EDMD) is also known as the condition called Humeroperoneal muscular dystrophy.
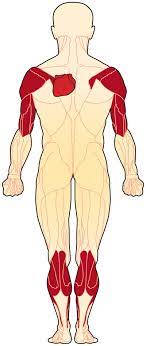
Emery-Dreifuss Muscular Dystrophy (EDMD) is one of the nine types of muscular dystrophy, a rare genetic degenerative disease that causes progressive impairment of skeletal muscle, which is the muscles used for movement and the electrical system of the heart. It causes weakness in the child’s upper arms, shoulders, and calves. It also causes joint stiffness which means the limb can not move well, known as contractures.
Symptoms are usually seen at about the age of 10 years and slowly get worse. EDMD generally affects boys, but girls also can get some forms of the disease.
The disease is named after Alan Eglin H. Emery and Fritz E. Dreifuss, physicians who first gave knowledge about the condition to a Virginia family in the 1960s.
Emery-Dreifuss Muscular Dystrophy (EDMD) can categorize into 3 different categories:
(1) X-linked EDMD
(2) Autosomal Dominant EDMD
(3) Autosomal Recessive EDMD
Prevalence of EDMD
It is rare condition which affects, 1 per 250,000 (0.39 per 100,000) people.
There are around 250,000 people in the United States affected by this type of muscular dystrophy.
The frequency of X-linked Emery Dreifuss Muscular Dystrophy approximately affects 1 in 100,000 people in the local population, and it is said to be the third most common type of muscular dystrophy. This type is fully seen in males only but, 10-20% of female carriers for X-linked EDMD will develop muscle weakness and/or heart conduction defects.
The autosomal recessive type of the disorder is extremely rare, with only a few cases worldwide. This type of EDMD affects females and males equally.
The frequency of autosomal dominant EDMD is not known.
What causes Emery-Dreifuss muscular dystrophy ?
Emery-Dreifuss muscular dystrophy (EDMD) has various forms. All are caused by mutations or changes in genes that make 3 proteins, which are emerin, lamin A, and lamin C in muscle cells. These proteins are one part of the membrane or envelope around the nucleus, which is the sac that contains the genes or chromosomes, in cells of the muscle. The defective gene is in X-linked EDMD. Muscles are made up of bundles of fibers or cells. Groups of proteins, which are present within the cell and in the membrane surrounding each fiber help keep muscle cells working properly and efficiently. When one of these proteins is absent or inadequate, which means a gene fails to make it properly; the result is one form of muscular dystrophy. A small protein named emerin is generally located in the membrane that surrounds each cell’s nucleus.
The loss of emerin from the nuclear cell membrane in X-linked EDMD leads to the symptoms of Emery-Dreifuss muscular dystrophy. This lack of emerin protein interferes with the resettlement of the nuclear membrane after a cell division, it leads to death or weak cells. Along with this, the gene that can be found defective in both the recessive and dominant autosomal forms of Emery-Dreifuss muscular dystrophy (EDMD) contains two closely related proteins called lamin C and lamin A that also are associated with the nuclear cells membrane. The nuclear cell membrane may become destabilized when the lamin proteins are defective, which leads to the breakdown of muscle.
Parents may pass down Emery-Dreifuss muscular dystrophy (EDMD) to their children in various ways:
(1) X-linked chromosome
X-linked is the most common form of Emery-Dreifuss Muscular Dystrophy (EDMD), which means that the changed gene or gene mutation is on the X chromosome. This type of EDMD is generally caused by a mutation or defect in the EMD gene, which helps in making the emerin protein. Emerin is useful for the normal function of cardiac and skeletal muscles.
Generally, boys are more potential to be affected by this form of Emery-Dreifuss Muscular Dystrophy (EDMD). Girls have two X chromosomes, one from every single parent. Males have 1 copy of the X chromosome from their mother and a Y chromosome from their father. So, a mutation means that he does not have enough of the protein to keep his muscles working properly. A female must have two mutated X chromosomes to have this form of Emery-Dreifuss Muscular Dystrophy (EDMD). A girl with a gene mutation generally gets enough normal protein from her other X chromosome; however, she can be a carrier for the mutation and can pass down it to her children. A female carrier is generally asymptomatic but is still at risk for cardiac conditions.
(2) Autosomal dominant
This type of EDMD affects both girls and boys, and one parent can pass down this form to her or his children. This type of inherited pattern is known as autosomal dominant. This means that only one copy of the defective LMNA gene, which is useful to make the proteins namely lamin A and lamin C; is enough to cause the disorder. It is not needed to be a family history of EDMD for this type of gene mutation to occur.
(3) Autosomal recessive
This is the rare form of EDMD that happens when a child inherits a mutated gene from both parents, known as autosomal recessive. Both girls and boys can have this type of EDMD. This form of EDMD occurs when a person has two copies of the altered or defective LMNA gene, one copy from each parent, which helps to make the proteins lamin C and lamin A. A person with only one gene mutation can be considered a carrier.
Symptoms of EDMD
Characteristic features of EDMD
It is identified by a classic triad of:
(1) Early Contractures in elbows, Achilles tendon, and posterior cervical muscles.
(2) Progressive atrophy of Humero-peroneal distribution which is proximal muscles of the upper limb and distal muscles of the lower limb. The proximal limb-girdle musculature also becomes weak in a long period of time.
(3) Cardiac conduction abnormalities.
Symptoms are usually seen by 10 years of age. Cardiac problems are generally diagnosed by 20 years of age. Females who are carriers of X-linked Emery Dreifuss Muscular Dystrophy (EDMD) would not have skeletal muscle involvement but can have cardiac conduction abnormalities.
Signs and Symptoms
- elbow contracture
- Humeral and peroneal muscle atrophy
- Symmetric weakness of triceps and biceps muscle with deltoids spare
- Toe walking
- Face, thigh, and hand weakness is uncommon and occurs later in the disease
- Atrioventricular block
- Atrial paralysis
- Cardiomyopathy, which may lead to heart failure
- Fainting or fluttering heartbeat means palpitations due to effect on heart
- Sudden death
Systemic Involvement
- The weakness is slow in progression and mainly affects the voluntary muscles that present over the humerus bone, which are the biceps and triceps muscles, and those lied on the outer surface of the lower leg that is peroneal muscles. Later on, the pelvic girdle and the scapular muscles can weaken, identify as a pattern that is termed as ‘Scapulohumeroperoneal.’ A winging of scapula caused by Weakness of the scapular fixators, which lead to impairment of the ability to lift the arms over the head and can be painful. Toe walking is caused by Weakness of the peroneal muscles, which is usually present in the first decade of life. Hand, Facial, and thigh muscles can be affected rarely. Hypertrophy of calf muscle can be seen.
- Contractures occur and it makes the neck, arm, ankle, and spine movements difficult or hard, which leads to a “toe-walking” pattern and difficulty in bending the elbows. Contractures are frequently seen before weakness and it can be more severe. In that, it tends to fix the ankle into plantarflexion via shortening of the Achilles tendon and elbow into flexion. The spine is also affected usually, with starting of limited neck flexion, and eventually, the whole spine can become fixed into extension, which is known as a rigid spine.
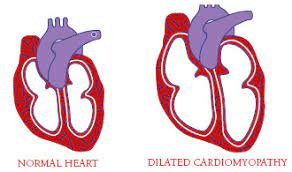
- Heart Involvement occurs in almost every case, first present as syncope in the second or third decades, otherwise as sudden cardiac death. A pacemaker is frequently required by 30 years of age due to a multitude of cardiac arrhythmias and Cardiac conduction deficits. Sometimes, cardiac involvement is the predominant feature of Emery Dreifuss Muscular Dystrophy (EDMD) with minor involvement of the skeletal muscles. Patients with Emery Dreifuss Muscular Dystrophy (EDMD) present with a variety of cardiac problems including atrioventricular conduction defect, atrial fibrillation or flutter, atrial standstill, atrial paralysis, congestive cardiac failure, poor exercise tolerance, bradycardia, syncope, and cardioembolic stroke. Later in the condition, dilated cardiac myopathy can occur. The people are at high risk for developing supraventricular tachyarrhythmias which are associated with a high risk for thromboembolic stroke and severe bradyarrhythmias which carries a risk of sudden death. It is possible for stroke to be the first clinical feature of Emery Dreifuss Muscular Dystrophy (EDMD).
Diagnosis of EDMD
Clinical Diagnosis
In the diagnosis of muscular dystrophy, a doctor basically immense by taking a history of patient and family and performing a physical examination. Generally, the origin including the pattern of the weakness can be pinpointed by a physical exam. The history and physical examination can make the diagnosis, even before any complicated special diagnostic tests are done.
The doctor also wants to diagnose whether the patient’s weakness results from a problem in the nerves that control them or in the muscles themselves. Problems with motor nerves, or muscle-controlling nerves, originate in the spinal cord and reach out to all the muscles, which can cause weakness that looks like a muscle problem but actually not.
The clinical diagnosis of Emery Dreifuss Muscular Dystrophy (EDMD) is done based upon the presence of the triad.
Nonspecific Laboratory Diagnosis
Early in the diagnostic procedures doctors generally order a special blood test known as a CK level, which stands for creatine kinase, an enzyme that leaks out from the damaged muscle.
Serum CK (creatine kinase) Concentration can be normal or mild to moderate elevation up to 2-20x upper normal level. Usually, rises are present more frequently at the starting of the condition as opposed to later stages.
While elevated CK levels are present in a blood sample, it generally means the muscle is destroyed by an abnormal process, like inflammation or muscular dystrophy. So, a high CK level often suggests the main cause of the weakness in the muscles themselves, but it can not tell exactly what the muscle disorder might be.
To check which disease is causing CK level raise, a doctor can suggest a muscle biopsy, that is the surgical removal of a tiny muscle sample from the patient with muscular dystrophy. By examination of this sample, a doctor can tell about what is actually doing inside the muscles. Muscle Histopathology shows nonspecific dystrophic or myopathic changes such as an increase in internal nuclei, variation in fiber size, increase in endomysial connective tissue, necrotic fibers. Electron Microscopy may identify specific changes in nuclear architecture. Modern techniques can use the biopsy to differentiate muscular dystrophies from inflammatory conditions, infections, and other issues. Muscle Biopsy is generally rarely used for diagnostic purposes because of lack of specificity.
Other tests on the biopsy sample can provide information regarding which muscle proteins are present in the muscle cells, and whether they are present in the perfect locations and in the normal amounts. By this, the doctor and patient can know what is wrong with the cells’ proteins and which genes are responsible for the problem.
Immunodetection of Emerin protein, which is detected by western blot and/or by immunofluorescence, is Absent in 95% of people with X-linked EDMD. Normally higher in patients with Autosomal Dominant EDMD.
Immunodetection of FHL1, which is detected by western blot and/or by immunofluorescence, is Absent or significantly decreased in patients with FHL1-related X-linked EDMD.
Genetic Testing
Genetic (DNA) testing with the use of a blood sample, can determine the presence of particular defects that cause Emery Dreifuss Muscular Dystrophy (EDMD) and can help predict the likely course of the condition, as well as help families assess the risk of passing the disease on to the next generation.
Other Nonspecific Clinical tests
Sometimes, special tests such as electromyography (EMG) and nerve conduction studies are done, which are uncomfortable but not generally very painful. In these, very fine pins and electricity are used to stimulate and assess the nerves or muscles specifically to see where the problem stays.
Findings of Electromyogram (EMG) usually suggest myopathic features with normal nerve conduction studies. While neuropathic patterns can be found in autosomal dominant EDMD and X-linked EDMD.
Findings of the CT scan show a diffuse pattern of involvement in muscles including the soleus, biceps, peroneal, gluteus, external Vasti, and paravertebral muscles. Findings in the posterior thigh and calf have been found in person with Autosomal Dominant EDMD.
Treatment of EDMD
Medical treatment
There is no particular management for Emery Dreifuss Muscular Dystrophy (EDMD). It is based on the individual symptoms of the patient.
Genetic counseling may be beneficial for the patient and his or her family.
Medications include:
Anti-thromboembolic drugs
Antiarrhythmic drugs
Medications that should be avoided include:
Depolarizing muscle relaxants; succinylcholine
Volatile anesthetic drugs; halothane, isoflurane
Surgery:
Orthopedic surgery includes treatment of scoliosis and the Release of contractures; However, contractures are likely to recur in some cases, and orthopedic surgery to correct contractures is usually not performed.
A heart transplant may be essential for end-stage heart failure conditions.
Physical therapy
Implantable cardioverter-defibrillator or Cardiac pacemaker for AV block and conduction disorder
Non-pharmacologic and Pharmacologic therapy for heart failure
Monitor:
Respiratory function
Annual cardiac assessment, which includes ECG and echocardiography
Cardiac care
Cardiac conduction block
In this, a floppy and dilated heart can not pump properly. This type of heart problem occurs when the rhythm of the heartbeat is disturbed because the electrical impulses poorly communicate between the lower and upper chambers of the heart. Conduction block may lead to sudden cardiac arrest and it can be life-threatening. By age 30, about all those patients with EDMD will have some form of investigational cardiac involvement or heart complication.
Cardiac problems can be life-threatening and they require treatment with medication or the insertion of a pacemaker, which is a small device placed under the skin that uses electrical impulses to help the heart beat normally. In this era, the problem is easy to detect with an electrocardiogram. A person with a diagnosis of EDMD should be monitored regularly for signs and symptoms of cardiac conduction block.
Cardiomyopathy
Along with cardiac conduction problems, many patients with Emery Dreifuss Muscular Dystrophy (EDMD) may develop dilated cardiomyopathy, which is an impairment in the heart muscle’s ability to pump blood around the body because it’s thinned out and dilated or floppy.
Medications such as angiotensin-converting enzyme inhibitors, blood thinners, and diuretics, can help in the improvement the heart function when the heart is impaired.
Severe cases of cardiac problems may require heart transplants.
Occupational therapy
A trained occupational therapist can help patients with dystrophy to adapt to their routine activities to compensate for the loss of muscle strength and power and to become independent in performing daily activities of life.
They can recommend and train patients for the use of assistive devices like foot and ankle braces to prevent leg deformities, wheelchairs to help with mobility, home modifications such as toilet bars, dressing aids, shower handles, and communication devices to improve interpersonal interaction.
Physiotherapy treatment
Physiotherapy for people with Emery Dreifuss Muscular Dystrophy (EDMD) is centered on treating the symptoms of the disease. Patients should visit physiotherapists as early as possible after being diagnosed. physiotherapy interventions will improve the patient’s quality of life.
Contractures
It develops in early childhood and is most commonly seen in Emery-Dreifuss muscular dystrophy (EDMD) and can worsen even if muscle strength does not change. Contracture prevention is not easy, but maintaining range of motion with physiotherapy can help to slow contracture development. Contractures released by surgical interventions are challenging because they can recur in progression.
Treatments:
- Active and passive stretching to prevent the worsening of contractures and increase flexibility
- Range of motion exercises can help to slow the development of deformity and joint stiffness
- Active and passive exercises to help maintain and build muscle strength
- Strengthening exercises in order to improve and maintain muscle strength
- Respiratory Techniques like respiratory muscle training assisted coughing, etc